- GENERAL INFORMATION
1.1 The Study is a pilot technology-enabled population health study conducted by HPB, which seeks to better understand the health behaviours and lifestyles of Singapore residents.
1.2 Data collected for the Study will be used by HPB to develop mass-personalisation programmes powered by machine learning and artificial intelligence engines, to benefit the wider population. This will in turn contribute to the development of health promotion policies and programmes in Singapore.
1.3 Each Participant will be issued with a smart wearable device (“Device”) that will be synchronised with the Participant’s smartphone. The Device will collect the Participant’s health data and information such as physical activity, heart rate and sleep that will be sent to hiSG’s mobile application (“hiSG App”). Participants will also be required to log the information of their meals and sleep and answer survey questions on hiSG App.
1.4 The duration of the Study is two (2) years commencing from the date on which the Participant provides his/her informed consent to participate in the Study (“Onboarding”). This will be referred to as the “Study Period”.
1.5 Each Participant will be issued the Device on the date of Onboarding.
1.6 The Participants’ compensation will commensurate with their contribution to the Study through loyalty points and other equivalent rewards.
- PARTICIPATION IN THE STUDY
Eligibility
2.1 All Singapore residents (i.e. Singapore Citizens and Permanent Residents, only) may participate in the Study.
2.2 Each Participant will require a smartphone with an operating system compatible with hiSG App and a Singapore-registered mobile number.
2.3 HPB may include additional eligibility criteria, such as but not limited to age range, for different cohorts of Participants.
2.4 HPB reserves the right to select the final list of Participants for the Study from the pool of interested registrants based on demographics and lifestyle indicators (“Selection”) to ensure a good mix of Participants for the analysis of data.
2.5 HPB reserves the right to discontinue the participation, at any point during the Study at its sole discretion, of any Participant who has been found to have provided false information upon registration of the study or whom HPB deems ineligible for participation. In such cases, the Participant shall be deemed to have withdrawn from the Study prematurely and shall be required to comply with the withdrawal procedure as prescribed in Section 4 of these T&Cs.
Commitment required for participation
2.6 By consenting to participate in the Study, the Participant agrees to meet the following minimum requirements of use (“Minimum Requirements”) throughout the Study Period:
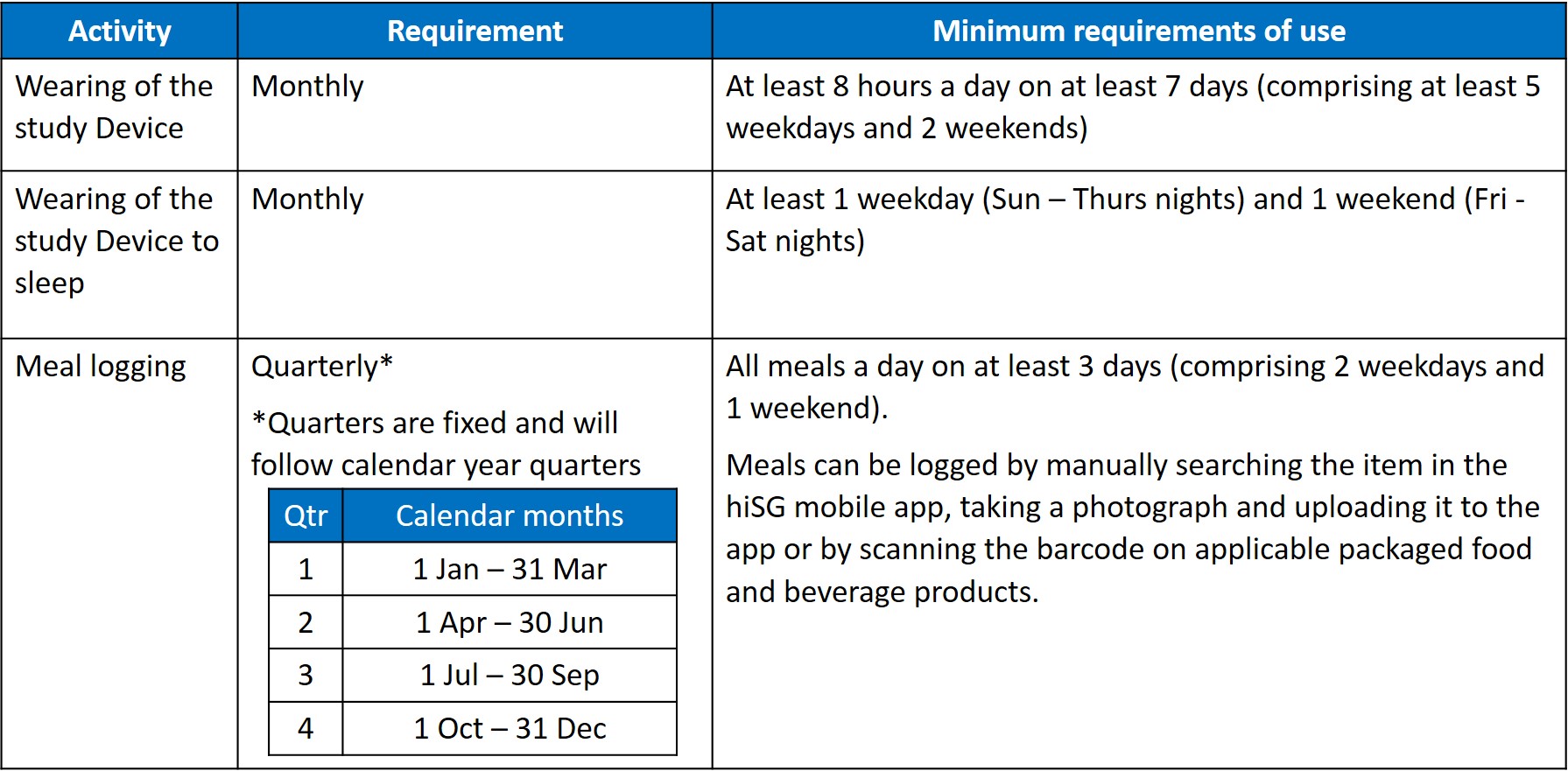
2.7 Should the Participant fail to meet any of the Minimum Requirements at any point during the Study Period, the Participant shall be deemed to have withdrawn prematurely from the Study. The Participant shall then be required to comply with the withdrawal procedure as specified in Section 4 of these T&Cs.
2.8 The Participant shall set up a Direct Debit Authorization arrangement with HPB upon Selection, thereby authorising HPB to collect payment from his/her bank account, as outlined in Schedule 1, should there be any outstanding payment owed by the Participant to HPB pursuant to these T&Cs.
2.9 The Participant shall pay Singapore Dollars fifty (S$50) upon his/her Onboarding as a deposit to receive the Device for his/her participation in the Study (“Deposit”). The Deposit shall be automatically refunded to the Participant in full upon his/her completion of the Study.
Compensation
2.10 The Participant shall earn “Healthpoints” for their participation in hiSG by:
- Wearing the Device
- Logging meals
- Logging sleep
- Responding to survey questions delivered on the mobile app and/or Device
2.11 HPB Healthpoints earned by the Participant will be accumulated and redeemable within hiSG App. All HPB Healthpoints accumulated under the Study will be consolidated with HPB Healthpoints earned from other HPB programmes and initiatives.
2.12 HPB reserves the right to determine and amend, at any point in time at its sole discretion, the number of HPB Healthpoints awarded for each activity in the Study.
2.13 HPB reserves the right to award HPB Healthpoints for additional tasks not specified in this document.
2.14 Any and all HPB Healthpoints awarded under the Study are strictly personal to the Participant only, and the Participant shall not transfer or assign his/her HPB Healthpoints to any other person.
2.15 By participating in the Study, the Participant also agrees to the “Terms of Use” applicable to the use of HPB Healthpoints found at www.healthhub.sg/rewards. The Terms of Use may be amended and/or modified from time to time at HPB’s sole discretion. The Participant’s continued participation in the Study thereafter represents his/her agreement to such amendments.
2.16 Please refer to Schedule 2 for more information about the rewards breakdown.
- STUDY DEVICE
3.1 For the purpose of the Study, HPB will be issuing each Participant with a study Device upon Onboarding. The Device and any accessories thereof (e.g. straps, charging cable) shall remain the property of HPB at all times throughout the Study Period.
3.2 Upon completion of the Study without early termination or withdrawal, the ownership of the Device shall be automatically transferred from HPB to the Participant with immediate effect. By way of illustration, if a Participant enrolls into the Study on 1 Aug 2018 and completes his/her participation on 31 July 2020, the ownership of the Device shall be fully transferred from HPB to that Participant on 1 August 2020. The Participant shall not be held liable or answerable to HPB for any matters relating to the Device and accessories thereof (e.g. loss, damage or sale of the Device and/or accessories thereof) from 1 Aug 2020. HPB likewise shall not be responsible for any matters relating to the Device and accessories thereof vis-à-vis the Participant from 1 Aug 2020. For the avoidance of doubt, if a Participant is withdrawn or terminated from the Study, for any reason whatsoever, prior to the expiry of the Study Period, the ownership of the Device shall remain with HPB in full and the Participant shall not have any right or ownership in the Device.
3.3 The Participant shall not exchange the Device with or loan, sell or cause the Device to be in the possession of other persons at any time throughout the Study Period. Upon Device issuance, the unique serial numbers of the Device shall be recorded under and linked to the Participant’s Fitbit account. HPB will be alerted should the issued Device be delinked or removed from the Participant’s registered Fitbit account.
3.4 HPB reserves the right to take legal action against Participants who exchange the Device with or loan, sell or cause the Device to be in possession of other persons at any time during the Study Period as the rightful owner of the Device and its accessories.
3.5 The Participant shall ensure that he/she is the sole user of the Device issued by HPB at all times throughout the Study Period and that all information provided by the Participant to HPB, including but not limited to user profile, demographics, responses to survey questions as well as logging of meals via hiSG App are true, accurate and based solely on their own information.
3.6 HPB reserves the right to terminate the participation of any Participant, at any point in time during the Study Period, who has been found at HPB’s sole determination to have not complied with Section(s) 3.3 and/or 3.5. The termination shall be deemed as withdrawal from the Study and the terms set out in Section 4 shall apply as if the Participant has withdrawn from the Study prematurely.
Faulty Devices
3.7 The Participant shall at all times during the Study Period exercise proper care of the Device and its accessories and adhere to the instructions of use and care as recommended by the manufacturer (as may be available on the user manual and/or manufacturer’s website).
3.8 Each Device shall be covered by a manufacturer’s warranty as provided by the manufacturer of the Device (“Device Warranty”) for two (2) years commencing from the respective date of issuance of the Device to the Participant (“hiSG Device Warranty Period”). The Device Warranty covers manufacturing defects only, such as defects in materials and workmanship. For the avoidance of doubt, the Device Warranty does not cover wear and tear, abuse or misuse and damage arising from the Participant’s fault, lack of care, negligence or failure to follow instructions relating to product use.
3.9 Should the Device be determined to be faulty due to manufacturing defects within the hiSG Device Warranty Period, the Participant may contact the manufacturer for direct replacement service against the Device Warranty.
3.10 For faulty Devices arising from wear and tear or the Participant’s lack of care, negligence, abuse, misuse, failure to follow instructions relating to product use or otherwise excluded from the manufacturing defects, the Participant shall pay for the cost of replacing the Device according to the rates set out in Schedule 1, as endorsed by HPB and the Participant at Onboarding.
Lost/Stolen Devices
3.11 In the event that the Device was lost or stolen, the Participant shall inform HPB immediately and follow the procedure as set out in Section 3.12 of these T&Cs. In the event that the Device was stolen, the Participant shall also make a police report and provide HPB with a copy of the report as soon as possible.
3.12 The Participant shall make payment to HPB for the replacement of the Device in accordance with the rates set out in Schedule 1, as endorsed by HPB and the Participant at Onboarding. Upon receipt of the aforesaid payment from the Participant, HPB shall issue a new Device for the Participant to resume participation in the Study.
3.13 Any extenuating circumstances may be considered by HPB on a case-by-case basis at HPB’s sole determination upon request by the Participant. Any decision made by HPB shall be final.
- PREMATURE WITHDRAWAL OF PARTICIPATION
4.1 The Participant shall be deemed as withdrawing from the Study with immediate effect upon occurrence of any of the following events:
a. The Participant notifies HPB in writing that he/she wishes to terminate his/her participation before the expiry of the Study Period;
b. The Participant fails to meet the Minimum Requirements as set out in Section 2.6 at any point during the Study Period;
c. HPB notifies the Participant of the termination of his/her participation due to fraud or unlawful acts; and/or
d. The Participant been found to breach any of the T&Cs at any point during the Study Period.
4.2 The Participant withdrawing from the Study shall:-
a. if the Participant does not wish to keep the Device for personal use upon withdrawal, return the Device within seven (7) days in its original working condition; or
b. if the Participant wishes to keep the Device for personal use upon withdrawal, pay to HPB the Device takeover fee based on the duration of participation in the study, in accordance with the rates set out in Schedule 1, as endorsed by HPB and the Participant at Onboarding. HPB shall collect the aforesaid fee via the Direct Debit Authorization set up by the Participant after Selection. Upon payment of the Device takeover fee, the Participant may keep the Device, and the applicable warranty period of the Device shall, with immediate effect, change to a one (1) year period commencing from the respective date on which HPB had issued the Device to the Participant (“Manufacturer’s Default Warranty Period”) instead of the Device Warranty Period. For the avoidance of doubt, the Participant acknowledges and agrees that the hiSG Device Warranty Period is applicable only insofar as the Participant continues to participate in the Study and the Manufacturer’s Default Warranty Period applies retrospectively in the event of the Participant’s withdrawal from the Study for any reason whatsoever. By way of illustration, a Participant who withdraws from the Study at the tenth (10th) month of the Study Period and chooses to keep the Device for personal use shall have a remaining Device warranty period of two (2) months only.
4.3 Should the Participant fail to return the Device or returns the Device but not in its original working condition upon withdrawal, he/she shall pay the Device takeover fee based on the duration of participation in the study, in accordance with Schedule 1.
4.4 In the event that the Participant withdraws from the Study before the expiry of the Study Period for any reason whatsoever, the Deposit or any part thereof shall NOT be refunded to the Participant irrespective of whether the Participant choses to keep the Device pursuant to Section 4.2 of these T&Cs.
4.5 All HPB Healthpoints and rewards earned and redeemed by the Participant during the course of participation in the Study prior to date of withdrawal shall not be affected or revoked by HPB.
4.6 Upon withdrawal, HPB will deactivate the Participant’s hiSG App account and will cease to have access to the Participant’s data.
4.7 Any and all data that have been collected by HPB pursuant to the Participant’s participation in the Study prior to the Participant’s withdrawal will continue to be kept by HPB and utilised for the purposes of the Study .
4.8 HPB reserves the right to take legal action against Participants who have withdrawn from the Study prior to the expiry of the Study Period but did not comply with any of the terms set out in these T&Cs.
- DATA PRIVACY AND CONFIDENTIALITY
5.1 All data and information provided by Participants and collected for the Study and/or during the Study Period shall be managed in accordance with the HPB’s Data Protection Policy. Please refer to www.hpb.gov.sg/data-protection-policy for more details.
5.2 By participating in the Study, the Participant consents to HPB’s collection, use and/or disclosure of all data collected by and/or transmitted through the Device via hiSG App. The Participant acknowledges and agrees that by linking and synchronising hiSG App with the Participant’s Device account, data from the Participant’s Device will be transmitted to hiSG App via the Device’s server.
5.3 By participating in the Study, the Participant acknowledges and agrees that his/her personal information submitted to or transmitted via hiSG App shall be treated in accordance with HPB’s Privacy Statement (as made available at https://www.hpb.gov.sg/privacy-statement) and these T&Cs, and that he/she shall be bound by the terms thereof. HPB reserves the right to change the terms of HPB’s Privacy Statement and/or these T&Cs at any time at its sole discretion. The Participant’s continued use of hiSG App represents the Participant’s agreement to any such amendments. If there is any conflict between HPB’s Privacy Statement and these T&Cs, these T&Cs will prevail.
5.4 By participating in the Study, the Participant consents to the collection, use and disclosure of his/her personal information by HPB for the purposes as set out herein. To safeguard the Participant’s personal data, HPB shall ensure that all electronic storage and transmission of personal data is secured with appropriate security technologies.
5.5 All rights to the information and data collected by or captured within hiSG App as well as all other data, statistics and/or analysis created or derived in connection with or as a result of the Participant’s participation in the Study shall vest in HPB.
5.6 HPB may use the data and/or information collected (including but not limited to the Participant’s personal information), in conjunction with other data sources, to formulate policies and/or programmes to promote health for the general public of Singapore.
5.7 HPB will ensure that only anonymised statistical information will be used for publication in academic or scientific journals.
5.8 HPB may share the data collected pursuant to the Study with other Government agencies, to the extent necessary to serve the Participant in a most efficient and effective way, unless such sharing is prohibited by law.
5.9 HPB shall not share the Participant’s personal data with non-Government entities, except where such entities have been authorised by HPB to carry out functions on HPB’s behalf for the purposes set out herein.
5.10 HPB will retain contact information provided by individuals during pre-registration so as to invite them to participate in future cohorts of the study where they are eligible for.
- OTHER TERMS AND CONDITIONS
6.1 HPB may vary the terms and conditions of these T&Cs and/or any other documents mentioned in herein without notice, or discontinue or withdraw the Study at any time without any further notice or liability to any Participant. In the event that HPB discontinues or withdraws the Study, the Participant shall not be deemed to have withdrawn prematurely from the Study and shall not be liable under Section 4.
6.2 The Participant agrees to abide by all the terms and conditions governing the use of hiSG App, which can be found embedded within the hiSG App.
6.3 To the fullest extent permitted by law, HPB shall not be liable for any injuries sustained/casualty that arises directly and/or indirectly from the Participant’s participation in the Study or use of hiSG App.
6.4 To the fullest extent permitted by law, HPB shall not be liable for or in respect of any expenses, losses, costs, damages, liabilities or other consequences of whatsoever nature (collectively “Losses”) suffered or incurred directly or indirectly by Participants, howsoever caused or arising and without limiting the generality of the foregoing, whether by reason of or on account of any act or omission whether negligent or otherwise on the part of HPB or its employees, directors, servants or agents, even if HPB or its employees, directors, servants or agents are advised of the possibility of such Losses.
6.5 HPB reserves the right to investigate cases of suspected fraud and suspend the Participant’s participation status and accumulation of HPB Healthpoints during the investigation period.
6.6 HPB reserves the right to terminate the participation of and/or withdraw compensation and/or rewards from any person who is in breach or not compliant with any of the terms of these T&Cs at its sole discretion.
6.7 The Participant agrees and consents to being contacted by HPB at any time to obtain feedback on the Study, the Device, hiSG App and/or any other matters related thereto.
6.8 By participating in the Study, the Participant agrees to be bound by and to comply with the T&Cs. HPB may, at any time, at its sole discretion and without prior notice or liability to the Participant, vary, modify and/or amend the terms within the T&Cs. Amendments will take effect when posted on the hiSG Webpage, and the Participant’s continued participation in the Study thereafter represents the Participant’s agreement to any such amendments.